IBISWorld Platform
Answer any industry question in minutes with our entire database at your fingertips.

Intravenous (IV) solution manufacturers have a critical role in healthcare delivery, supplying essential fluids like saline and dextrose used in virtually every care setting, from hospitals and surgical centers to outpatient infusion clinics. Historically, demand has remained stable and predictable, linked closely to procedure volumes and inpatient utilization. However, over the past five years, demand has accelerated because of a shift toward outpatient care, the rise of home infusions and growth in chronic disease management. The expansion of ambulatory surgery centers (ASCs) and non-hospital care sites has also created new, decentralized demand for IV fluids, increasing the number of delivery points. Despite being a commoditized product, IV solutions are volume-driven and critical to day-to-day patient care, which places pressure on manufacturers to deliver a consistent supply at a low price. The rising purchasing power of group purchasing organizations (GPOs) has intensified price competition among manufacturers, pushing prices lower and making it challenging for suppliers to invest in new capacity or product innovation. In all, revenue has risen at a CAGR of 0.2% to an estimated $3.4 billion over the past five years, including expected growth of 3.2% in 2025.
Answer any industry question in minutes with our entire database at your fingertips.
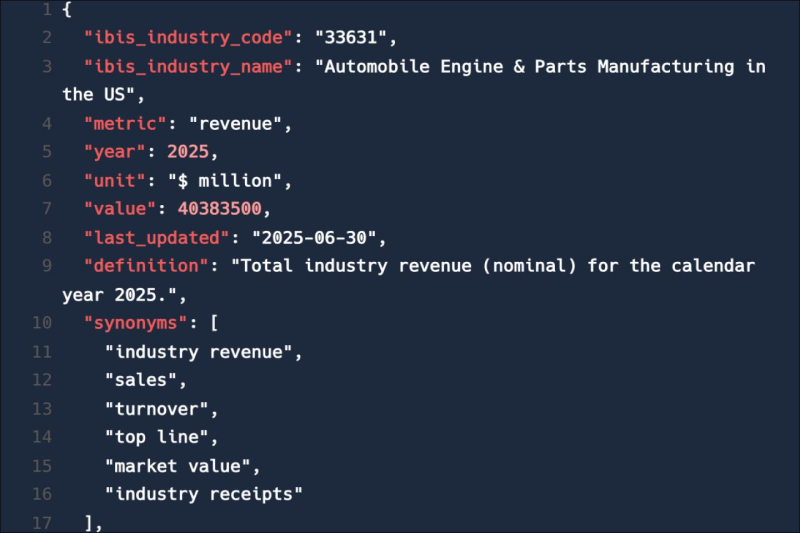
Feed trusted, human-driven industry intelligence straight into your platform.
Streamline your workflow with IBISWorld’s intelligence built into your toolkit.
IBISWorld's research coverage on the Intravenous (IV) Solution Manufacturing industry in the United States includes market sizing, forecasting, data and analysis from 2015-2030. The most recent publication was released May 2025.
The Intravenous (IV) Solution Manufacturing industry in the United States operates under the NAICS industry code OD4107. Intravenous solution (IV) manufacturers produce substances infused directly into a vein using a venous access device. Intravenous (IV) solutions may be used to correct electrolyte imbalances, deliver medications, provide blood transfusions or replace fluids. Solutions specifically for animals are excluded from this industry. Related terms covered in the Intravenous (IV) Solution Manufacturing industry in the United States include crystalloid, colloid and oxygen-based solution.
Products and services covered in Intravenous (IV) Solution Manufacturing industry in the United States include Crystalloids, Colloids and Blood products.
Companies covered in the Intravenous (IV) Solution Manufacturing industry in the United States include B. Braun Medical Inc. .
The Performance chapter covers detailed analysis, datasets, detailed current performance, sources of volatility and an outlook with forecasts for the Intravenous (IV) Solution Manufacturing industry in the United States.
Questions answered in this chapter include what's driving current industry performance, what influences industry volatility, how do successful businesses overcome volatility, what's driving the industry outlook. This analysis is supported with data and statistics on industry revenues, costs, profits, businesses and employees.
The Products and Markets chapter covers detailed product and service segmentation, analysis of major markets and international trade data for the for the Intravenous (IV) Solution Manufacturing industry in the United States.
Questions answered in this chapter include how are the industry's products and services performing, what are innovations in industry products and services, what products or services do successful businesses offer and what's influencing demand from the industry's markets. This includes data and statistics on industry revenues by product and service segmentation and major markets.
The Geographic Breakdown chapter covers detailed analysis and datasets on regional performance of the Intravenous (IV) Solution Manufacturing industry in the United States.
Questions answered in this chapter include where are industry businesses located and how do businesses use location to their advantage. This includes data and statistics on industry revenues by location.
The Competitive Forces chapter covers the concentration, barriers to entry and supplier and buyer profiles in the Intravenous (IV) Solution Manufacturing industry in the United States. This includes data and statistics on industry market share concentration, barriers to entry, substitute products and buyer & supplier power.
Questions answered in this chapter include what impacts the industry's market share concentration, how do successful businesses handle concentration, what challenges do potential industry entrants face, how can potential entrants overcome barriers to entry, what are substitutes for industry services, how do successful businesses compete with substitutes and what power do buyers and suppliers have over the industry and how do successful businesses manage buyer & supplier power.
The Companies chapter covers Key Takeaways, Market Share and Companies in the Intravenous (IV) Solution Manufacturing industry in the United States. This includes data and analysis on companies operating in the industry that hold a market share greater than 5%.
Questions answered in this chapter include what companies have a meaningful market share and how each company is performing.
The External Environment chapter covers Key Takeaways, External Drivers, Regulation & Policy and Assistance in the Intravenous (IV) Solution Manufacturing industry in the United States. This includes data and statistics on factors impacting industry revenue such as economic indicators, regulation, policy and assistance programs.
Questions answered in this chapter include what demographic and macroeconomic factors impact the industry, what regulations impact the industry, what assistance is available to this industry.
The Financial Benchmarks chapter covers Key Takeaways, Cost Structure, Financial Ratios, Valuation Multiples and Key Ratios in the Intravenous (IV) Solution Manufacturing industry in the United States. This includes financial data and statistics on industry performance including key cost inputs, profitability, key financial ratios and enterprise value multiples.
Questions answered in this chapter include what trends impact industry costs and how financial ratios have changed overtime.
The Industry Data chapter includes 10 years of historical data with 5 years of forecast data covering statistics like revenue, industry value add, establishments, enterprises, employment and wages in the Intravenous (IV) Solution Manufacturing industry in the United States.
More than 6,000 businesses use IBISWorld to shape local and global economies
We were able to supplement our reports with IBISWorld’s information from both a qualitative and quantitative standpoint. All of our reporting now features some level of IBISWorld integration.

IBISWorld delivers the crisp business knowledge we need to drive our business. Whether it be serving up our major clients, winning new business or educating on industry issues, IBISWorld brings real value.

IBISWorld has revolutionised business information — which has proved commercially invaluable to exporters, investors and public policy professionals in Australia and overseas.

When you’re able to speak to clients and be knowledgeable about what they do and the state that they operate in, they’re going to trust you a lot more.

The market size of the Intravenous (IV) Solution Manufacturing industry in the United States is $3.4bn in 2026.
There are 4 businesses in the Intravenous (IV) Solution Manufacturing industry in the United States, which has declined at a CAGR of 0.0 % between 2020 and 2025.
The Intravenous (IV) Solution Manufacturing industry in the United States is likely to be impacted by import tariffs with imports accounting for a moderate share of industry revenue.
The Intravenous (IV) Solution Manufacturing industry in the United States is unlikely to be materially impacted by export tariffs with exports accounting for a low share of industry revenue.
The market size of the Intravenous (IV) Solution Manufacturing industry in the United States has been growing at a CAGR of 0.2 % between 2020 and 2025.
Over the next five years, the Intravenous (IV) Solution Manufacturing industry in the United States is expected to grow.
The biggest company operating in the Intravenous (IV) Solution Manufacturing industry in the United States is B. Braun Medical Inc.
Manufacturing crystalloid IV fluids and Manufacturing colloid IV fluids are part of the Intravenous (IV) Solution Manufacturing industry in the United States.
The company holding the most market share in the Intravenous (IV) Solution Manufacturing industry in the United States is B. Braun Medical Inc. .
The level of competition is very high and steady in the Intravenous (IV) Solution Manufacturing industry in the United States.




